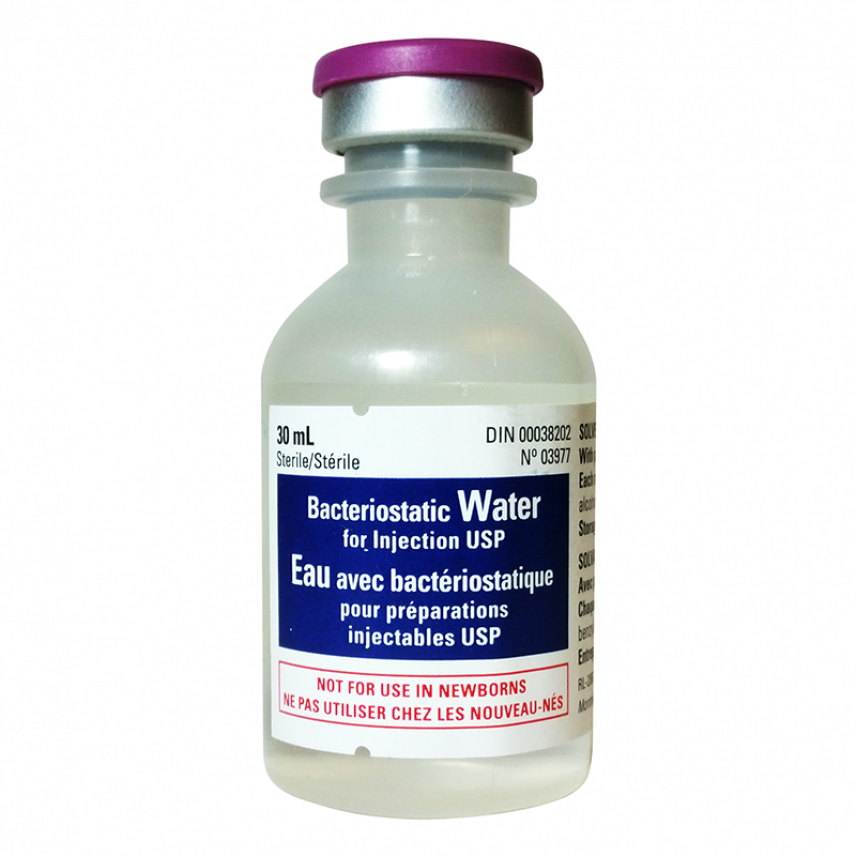
Bacteriostatic Water 30mL
$15.00
-

bacteriostatic water 30ml Bacterial water for Injections
- Must have to mix HGH/HCG
- Ideal for cutting bacteriostatic water 30ml.
Bacteriostatic Water 30ml: Complete Guide to Uses, Benefits, and Safety
Sterile solutions play a critical role in healthcare, research, and pharmaceutical handling. One such solution is bacteriostatic water 30ml. It is widely recognized for its preservative properties and its role in dilution and reconstitution contexts under professional supervision.
In this guide, you will learn what bacteriostatic water is, why the 30ml format is popular, how it differs from other sterile waters, and which safety and quality factors matter most. Moreover, the content focuses on clarity, compliance, and responsible information.
What Is Bacteriostatic Water?
Bacteriostatic water is sterile water that contains a small amount of benzyl alcohol, usually 0.9%. This additive helps prevent the growth of bacteria after the vial has been opened.
Unlike plain sterile water, bacteriostatic water supports multiple withdrawals when handled correctly in clinical or laboratory environments. Therefore, it is commonly used in professional healthcare and research settings.
Understanding the 30ml Format
The bacteriostatic water 30ml. is one of the most commonly available formats. Its popularity comes from convenience and reduced waste.
Why 30ml Is Widely Used
-
Suitable for repeated access under sterile conditions
-
Easier to store and transport
-
Efficient for clinics and research labs
Because of these advantages, bacteriostatic water 30ml often appears in medical supply inventories.
How Bacteriostatic Water Differs From Sterile Water
Although both are sterile, they serve different purposes.
Bacteriostatic Water
-
Contains benzyl alcohol
-
Helps inhibit bacterial growth
-
Intended for controlled, multi-use access
Sterile Water for Injection
-
Contains no preservatives
-
Designed for single use
-
Discarded after opening
As a result, choosing the correct solution depends on professional requirements and intended application.
Common Uses of Bacteriostatic Water 30ml
Bacteriostatic water has a narrow but important role.
Dilution and Reconstitution
Healthcare professionals often use it to dilute or reconstitute certain medications or compounds when permitted by labeling and guidelines.
Laboratory and Research Settings
In controlled environments, it supports preparation processes that require sterile conditions.
Clinical Convenience
Because it resists bacterial growth, it allows flexibility compared to preservative-free options.
It is important to note that all use depends on professional direction and product labeling.
Why Benzyl Alcohol Matters
Benzyl alcohol acts as a bacteriostatic water 30ml., meaning it slows or prevents bacterial growth rather than killing bacteria outright.
Benefits of Benzyl Alcohol
-
Extends usability after opening
-
Reduces contamination risk when handled properly
-
Supports multi-dose access
However, benzyl alcohol is not suitable for all populations or applications. Therefore, professional judgment remains essential.
Safety Considerations and Warnings
Safety is critical when handling sterile solutions.
Population Restrictions
Bacteriostatic water is not recommended for newborns or premature infants due to benzyl alcohol sensitivity.
Professional Use Only
Use should always follow medical or laboratory guidance. Misuse may lead to contamination or adverse outcomes.
Not a Medication
Bacteriostatic water does not treat disease. It serves as a diluent or preparation aid only.
Clear understanding prevents misuse and improves outcomes.
Storage and Handling Guidelines (General Information)
Proper storage preserves sterility and quality.
Storage Best Practices
-
Store at controlled room temperature
-
Protect from excessive heat and light
-
Keep vial sealed when not in use
Handling Awareness
-
Use sterile technique as required
-
Avoid contact with non-sterile surfaces
These general principles apply across clinical and research environments.
Shelf Life and Stability
Shelf life depends on manufacturing standards and storage conditions.
Unopened Vials
Remain stable until the manufacturer’s expiration date when stored correctly.
After Opening
Usability depends on professional guidelines and institutional protocols. Always follow official recommendations.
Never use solutions that appear cloudy, discolored, or compromised.
Quality Standards and Manufacturing
High-quality bacteriostatic water meets strict production standards.
What to Look For
-
Sterile manufacturing processes
-
Clear labeling and lot numbers
-
Compliance with pharmaceutical standards
Reputable suppliers emphasize transparency and quality assurance.
Regulatory and Legal Considerations
Regulations vary by country and region.
-
Often classified as a medical or pharmaceutical supply
-
Distributed through licensed suppliers
-
Subject to healthcare regulations
Before purchase, buyers should ensure compliance with local laws and institutional policies.
Bacteriostatic Water vs Saline Solutions
Another common comparison involves saline.
Bacteriostatic Water
-
Contains benzyl alcohol
-
No electrolytes
Bacteriostatic Saline
-
Contains benzyl alcohol
-
Includes sodium chloride
Each serves different professional needs. Therefore, selection depends on specific requirements.
Common Misconceptions
“It Is the Same as Sterile Water”
This is incorrect due to the preservative content.
“It Can Be Used for Anything”
Use depends on labeling, compatibility, and professional guidance.
“It Eliminates All Bacteria”
Bacteriostatic agents inhibit growth but do not sterilize contaminated solutions.
Understanding these distinctions improves safe handling.
Frequently Asked Questions (FAQ)
What does bacteriostatic water 30ml mean?
It refers to a bacteriostatic water 30ml. sterile water containing benzyl alcohol to inhibit bacterial growth.
Is bacteriostatic water reusable?
It supports multiple withdrawals under proper sterile conditions, according to guidelines.
Is bacteriostatic water the same as saline?
No. Saline contains sodium chloride, while bacteriostatic water does not.
Who should not use bacteriostatic water?
Newborns and premature infants should not be exposed to benzyl alcohol.
SEO Insight: Search Intent Behind “Bacteriostatic Water 30ml”
This keyword reflects commercial-informational intent. Users want:
-
Product understanding
-
Safety clarity
-
Quality assurance
Therefore, educational content paired with compliance improves both trust and SEO performance.
Ethical Distribution and Responsible Information
Ethical supply includes:
-
Accurate labeling
-
No exaggerated claims
-
Clear safety warnings
Responsible information protects end users and supports professional standards
BACTERIOSTATIC WATER
bacteriostatic water 30ml.- is a substance that is used by people in order to manufacture pharmaceutical products of liquid forms. This water includes 0.9% of benzyl alcohol 9mg / 1ml.
WHAT IS A BACTERIOSTATIC WATER: ITS QUALITIES AND DESCRIPTION AS A SOLVENT
Usually, this solvent is used in medicine and sports practice for making injection or infusion solutions, to achieve the best conditions for the effectiveness and consistency of water and substrates.
In sports such as bodybuilding, bacteriostatic water 30ml. is used for dilution of pharmacological agents, such as peptides, and in medicine – for the making pharmaceutical solutions.
Bacteriostatic water has some advantages which its counterparts (saline solution, sterile water, etc.) do not posses. First of all, the presence of benzyl alcohol in bacteriostatic water should be noted. It destroys all kinds of bacteria known to science, thereby protecting solution from their penetration and distribution).
While piercing the rubber cap of vial, you may insert harmful bacteria in solution which normally quickly multiply in sterile water. Injections that are influenced by bacteria’s actions may cause various infections.
BACTERIOSTATIC WATER: INSTRUCTION AND METHOD OF USAGE
Method of bacteriostatic water’s usage largely depends on the drug which will be dissolved on its base (expiration date of solutions and their dosages may vary depending on the substance). For example, if we dissolve the peptide GHRP-2 or Gonadorelin, the expiration date will be 30-35 days. If we dissolve Selank peptide, then its expiration date will be no more than 30 days, which is much more than use of saline solution or sterile water.
As for dilution, it should be mentioned that almost all pharmacological substances have universal instruction concerning bacteriostatic water. First, wipe vials with drug and with “solvent” alcohol. Then input the needed amount of solution with insulin syringe into the vial.
Make sure that the water for injection flows evenly along the walls of the ampoule. Wait for total dissolution of the solvent and the drug (it is not recommended to shake the vial in order to speed up the process), then you can make the injection


.
Be the first to review “Bacteriostatic Water 30mL” Cancel reply
Related products
Syringes & Accessories
Syringes & Accessories
Syringes & Accessories
Syringes & Accessories
Syringes & Accessories
Syringes & Accessories
Syringes & Accessories










Reviews
There are no reviews yet.